

In a mass where the free fat merely fills the small interstices between globules and crystals, the texture will be largely that of the separate particles. The difference is a matter of both large-scale and molecular arrangements. A preponderance of free fat, on the other hand, makes for a malleable butter that softens readily and may even weep some liquid fat in the process.

The more fat there is in discrete globules or crystals, the harder and more crumbly the butter, even to the point of brittleness. The proportion of continuous or "free" fat can vary from 50% of the total to nearly 100%, and it has a direct influence on the behavior of butter. The continuous, amorphous phase of solid fat surrounds not only the water droplets, but also air bubbles, intact fat globules, and highly ordered crystals of milk fat that have grown during the cooling process. The physical structure of butter is, however, a bit more complicated. The final product is about 80% milk fat, 18% water, and 2% milk solids, mainly proteins and salts carried in the water. The system of fat droplets dispersed in water is converted into a continuous phase of fat that contains water droplets. The process of butter making can be described as an inversion of the original cream emulsion. The fat globule membrane is comprised of surface active materials: phospholipids and lipoproteins.įat globules typically aggregate in three ways: Churning continues until the butter granules are about the size of wheat grains.įat globules vary from 0.1 - 10 micon in diameter.
Added water is necessary to help the cream to 'break' but the water should not exceed 25% of the total volume of cream. Cold water at 10☌ is then added and then it is agitated again. Paddles slowly agitate the cream causing it to thicken and separate into butter grains and buttermilk. As churning continues, then, the foam gradually subsides, and the butter granules are worked together into larger and larger masses. These materials disrupt thin water layers and so burst bubble walls, and once enough of them have been freed in the process of whipping or churning cream, the foam will never be stable again. The foam structure is broken both by the free fat and the released membrane materials, which include emulsifiers like lecithin. Persistent agitation knocks the softened globules into each other enough to break through the protective membrane, and liquid fat cements the exposed droplets together. The ideal temperature range is said to be 55° to 65☏ (12° to 18☌).

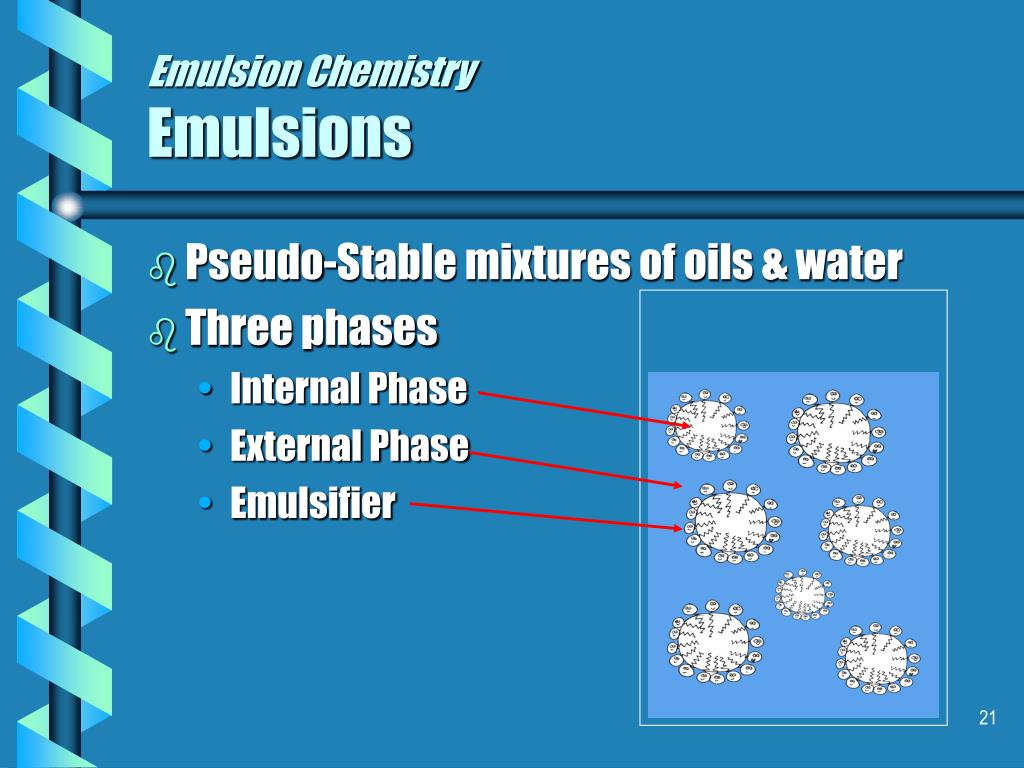
But where whipping cream is kept cold, and the agitation stopped when a a stable, airy foam is produced, churned cream is warmed to the point that the globules soften and to some degree liquify. Current theory runs along these lines: just as happens in whipped cream, some air is incorporated into the liquid, bubbles form, and the fat globules collect in the bubble walls. In four glasses or test tubes place 2.5ml vinegar and 2.Exactly how churning works is still unknown.Emulsions are thicker than either the water or of fat/oil they contain, which is a useful property for some foods. By vigorously mixing the emulsifier with the water and fat/oil, a stable emulsion can be made.Ĭommonly used emulsifiers include egg yolk, or mustard. The hydrophilic end of the emulsifier molecule is attracted to the water and the hydrophobic end is attracted to the fat/oil. These help to form and stabilise the emulsions, preventing or slowing the water and fat/oil from separating.Įmulsifier molecules work by having a hydrophilic end (water-loving) and hydrophobic end (water-hating). To prevent the mixture from separating substances called emulsifiers can be added. However the mixture is unstable and if you left it for a while it would soon separate out into water and oil layers again. If you shake the oil and water together then the oil breaks up into tiny droplets and becomes distributed in the water forming a mixture. If you add a drop or two of oil to water you can see that it does not dissolve or combine with the water: the oil floats on the water.


 0 kommentar(er)
0 kommentar(er)
